BACKGROUND: Acute myelogenous leukemia (AML) cells that reside in bone marrow (BM) receive a great deal of protection from the cytotoxic effects of therapeutic agents. In contrast, the circulating leukemia cells are typically more chemosensitive compared to those embedded in BM niches. The BM homing of AML cells is mediated by multiple adhesive and chemokinetic interactions including, respectively, by sialylated glycoproteins on the cancer cells binding to E-selectin on the endothelium and by CXCR4-mediated sensing of SDF-1/CXCL12 gradients. Consequently, both E-selectin and CXCR4 inhibitors have been pursued as targets for add-on therapeutic strategies aimed to "mobilize" AML cells out of the BM. A next generation "mobilizing" agent GMI-1359 combines, in one molecular structure, the E-selectin and CXCR4 inhibitory moieties. In a previous study, GMI-1359 markedly reduced leukemia cell adhesion to endothelial cells, leukemia cellularity in BM (Zhang et al., 2016) and it enhanced the survival of cytarabine/daunorubicin therapy in a FLT3-mutated AML model (Zhang et al., 2015).
APPROACH: Here, we used intravital 2-photon microscopy of calvarial BM to study the behavioral response of AML cells to the dual CXCR4 and E-selectin inhibition (GMI-1359) in vivo. We used a mTurquoise2 fluorescence-tagged transplantable mouse AML model (characterized by MLL, ENL-FLT3, ITD, p53-/-). To delineate the bone and vascular niches, the syngeneic immune-competent recipient mice harbored cell lineage fluorescence reporter genes such as Col2.3-GFP, hCD2-DsRed and CD11c-EYFP with the bone collagen and blood highlighted by, respectively, second harmonic generation (SHG) and fluorescent dextran. Intravenously infused AML cells (5x10E4) homed to BM where they gradually displaced most endogenous cells. In this experimental system, GMI-1359 or vehicle were infused into the tail vein while recording AML cell motilities in calvarial BM stroma in 3-D over 4 hours.
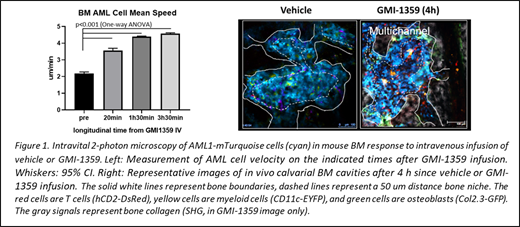
RESULTS: In untreated or control vehicle treated mice, AML cells were slowly motile, migrating with the average velocity of ~2 µm/min. Cellular trajectories were random within the average 400 µm confinement radius corresponding to the size of BM cavities. Most AML cells localized in the vascular niche, defined as within a 50 µm distance to the nearest blood vessel. A minority of AML cells were also present in the proximity to osteoblasts and the bone (i.e. in the bone niche). In contrast, GMI-1359 infusion resulted in a 66% increase of the average speed of cellular motility of AML cells within 20 min and up to 100% increase within 3.5 hours. The motility pattern remained largely random within the bone cavity confinement volume and was concentrated in the vicinity of vasculature. Thereafter, we observed multiple instances of AML cell intravasation into the capillary lumens followed by several minute-long vessel wall attachment and sudden outflow. This cellular dynamics resulted in a substantial, yet structurally biased decrease of BM AML cellularity whereby the vascular niche was emptied preferentially whilst the bone niche remained populated by AML cells. In no case we observed a complete BM depletion of AML cells. Interestingly, as we reported before, a single, CXCR4-only inhibitor caused a protracted (24-48 h) depletion of AML cells in BM without significant cellular motility enhancement.
CONCLUSIONS: Our observations reveal an unexpected mechanism of action by a dual E-selectin/CXCR4 inhibitor involving cellular migratory motility enhancement in BM stroma prior to AML cell intravasation. We envisage that, besides the designed blocking of E-selectin and CXCR4 molecules from binding their corresponding ligands, the dual moiety GMI-1359 agent has a capacity to generate motility-enhancing signals in AML cells that single inhibitors do not seem to trigger. Among several mechanisms possible, this action could involve E-selectin and CXCR4 crosslinking by a dual moiety molecular structure or binding avidity enhancement, requiring further investigation. In addition, our results highlight a likely mechanism of AML resistance to therapeutic "mobilization", one that involves cancer cell persistence in the bone/osteoblastic niches.
Zal:Daiichi-Sankyo: Research Funding; Moleculin Biotech, Inc.: Research Funding. Fogler:GlycoMimetics: Current Employment, Current equity holder in publicly-traded company, Patents & Royalties. Magnani:GlycoMimetics, Inc.: Current Employment, Current equity holder in publicly-traded company, Membership on an entity's Board of Directors or advisory committees, Patents & Royalties. Andreeff:Daiichi-Sankyo; Jazz Pharmaceuticals; Celgene; Amgen; AstraZeneca; 6 Dimensions Capital: Consultancy; Daiichi-Sankyo; Breast Cancer Research Foundation; CPRIT; NIH/NCI; Amgen; AstraZeneca: Research Funding; Amgen: Research Funding; Centre for Drug Research & Development; Cancer UK; NCI-CTEP; German Research Council; Leukemia Lymphoma Foundation (LLS); NCI-RDCRN (Rare Disease Clin Network); CLL Founcdation; BioLineRx; SentiBio; Aptose Biosciences, Inc: Membership on an entity's Board of Directors or advisory committees.
Author notes
Asterisk with author names denotes non-ASH members.

This feature is available to Subscribers Only
Sign In or Create an Account Close Modal